The increase in depression is most correlated with the increase in inflammatory autoimmune diseases and with significant changes in the western diet.
The US suicide rate has increased by 40% over the last 20 years.
The increase in suicides that appeared around the year 2000 correlates highly with changes in the American diet starting around the year 2000.
One such change is the increase in vegetable oil consumption. This vegetable oil is often used in fried foods and contains many pro-inflammatory trans-fats.
The rise in suicides also correlates with a 10% decrease in vegetable intake. Vegetables themselves are generally anti-inflammatory.
The rise in suicides also correlates with a 25% decrease in fruit intake. Fruit is also typically anti-inflammatory.
The prevalence of autoimmune disorders has been increasing for many decades.
If an individual has an autoimmune disease they are much more likely to also have depression and vice versa.
Depression has all of the hallmarks of sickness behavior triggered by the immune system when people are sick by inhibiting the production of serotonin and melatonin.
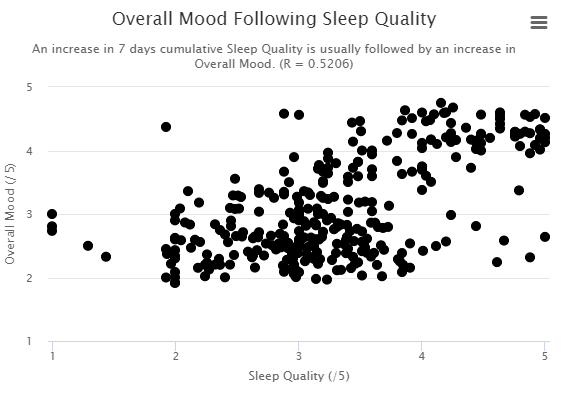
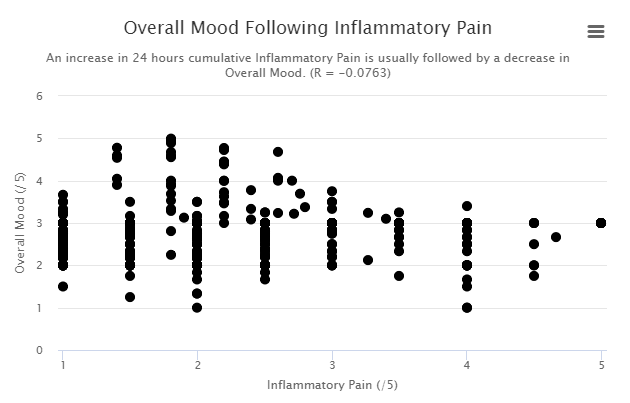
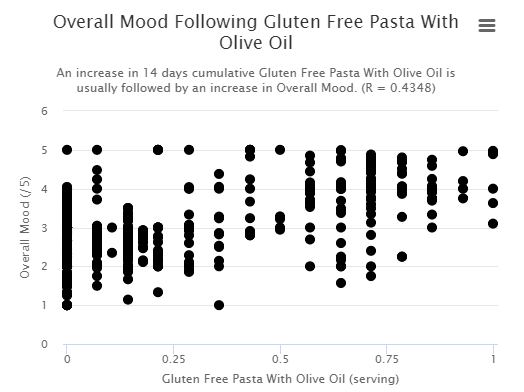
As shown above, our new diet is highly inflammatory so it’s triggering this sickness behavior all the time for many people.
Crosstalk Between Inflammation and Glutamate System in Depression: Signaling Pathway and Molecular Biomarkers for Ketamine’s Antidepressant Effect
Conventional antidepressants are ineffective for more than 30% of patients. In such patients, who have what is called treatment-resistant depression (TRD), inflammatory biomarkers are expressed excessively in both the central nervous system (CNS) and the peripheral blood. Ketamine, a glutamate receptor antagonist, exerts a rapid and sustained therapeutic effect in patients with TRD. Thus, the investigation of the relations between inflammation and glutamate underlying depression has drawn great attention. Inflammation influences glutamate release, transmission, and metabolism, resulting in accumulated extracellular glutamate in the CNS. Downstream of the glutamate receptors, the mammalian target of rapamycin (mTOR) signaling pathway plays a key role in mediating ketamine’s antidepressant effect by improving neurogenesis and plasticity. Based on the mechanism and clinical evidence of the inflammatory contribution to the pathogenesis of depression, extensive research has been devoted to inflammatory biomarkers of the clinical response of depression to ketamine.
Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice
The purpose of the present study was to investigate whether ketamine’s rapid antidepressant effects were associated with its anti-inflammatory actions and to explore the underlying molecular mechanism. Depressive-like behaviors was induced in mice using chronic restraint stress (CRS) method. Anti-depressive effects of ketamine were evaluated by forced swimming tests (FST) and sucrose preference test (SPT). Subsequently, brain tissue was harvested to investigate inflammatory response in the hippocampus via investigating reactive microglia numbers, serum cytokines levels and the toll-like receptor type 4 (TLR4)/p38 mitogen-activated protein kinase (MAPK) pathway. CRS exposure caused depressive-like behaviors in mice, which was associated with increased pre-inflammatory cytokines (interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6) levels, reactive microglia numbers and up-regulated regulatory molecules such as TLR4/p38 and P2X7 receptor in hippocampus. Such neurobehavioral and biochemical abnormalities were normalized by ketamine treatment. CRS-induced depression-like behaviours are associated with activation of hippocampal inflammatory response, whereas down-regulation of pro-inflammatory cytokines may contribute to ketamine’s antidepressant effects in mice.

Leave a Reply