In the 1800s there was a big problem with snake oil salesmen making false claims about the concoctions they were selling. The original solution was the AMA Council on Pharmacy and Chemistry certification to ethical drug products that met their standards.
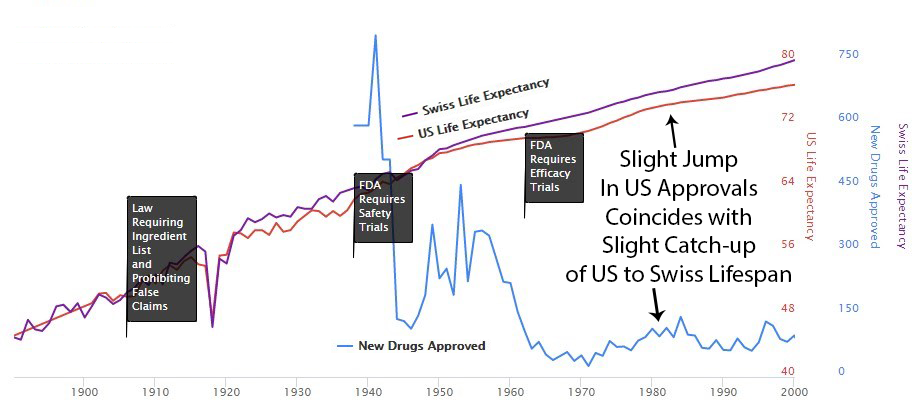
1906 – Ingredient Lists and No Lying on Labels
The 1906 Pure Food and Drugs Act empower the Bureau of Chemistry (forerunner of the FDA) to seize adulterated and misbranded products. The law also prohibited “false and misleading” statements on product labels. The law listed eleven so-called “dangerous ingredients” including opium (and its derivatives) and alcohol which, if they were present in the product, had to be listed on the drug label. This listing requirement alone inspired many manufacturers to abandon the use of many “dangerous ingredients” following the passage of the 1906 Act.
1938 – Safety Trial Requirements
The Federal Food, Drug, and Cosmetic (FDC) Act of 1938 is passed by Congress, containing new provisions:
- Extending control to cosmetics and therapeutic devices.
- Requiring new drugs to be shown safe before marketing
Manufacturers were required to demonstrate to FDA that they had carried out all reasonably applicable studies to demonstrate safety and that the drug was “safe for use”.
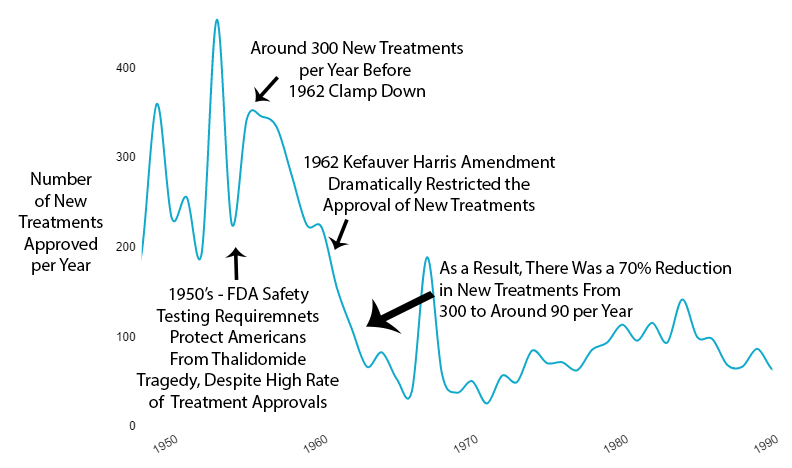
1962 – New Treatment Approvals Cut By 70%
In the late 1950s, the drug thalidomide became available in 46 countries (but not the US) to treat nausea associated with morning sickness during pregnancy. Unfortunately, the potential side effects were not fully understood. As a result, thousands of children were born with birth defects, most notably phocomelia (limb malformations).

Fortunately, the existing 1938 FDA safety requirements completely protected Americans from the fate of Europe. However, amid the mass hysteria over the tragedy, the US government felt compelled to look like it was doing something in response.
As effective safety regulations were already in place, the government instead chose to restrict the production of new treatments primarily by requiring extensive additional efficacy testing via the 1962 Kefauver Harris Amendment.
Results of Increased Restrictions
Reduction in New Treatments
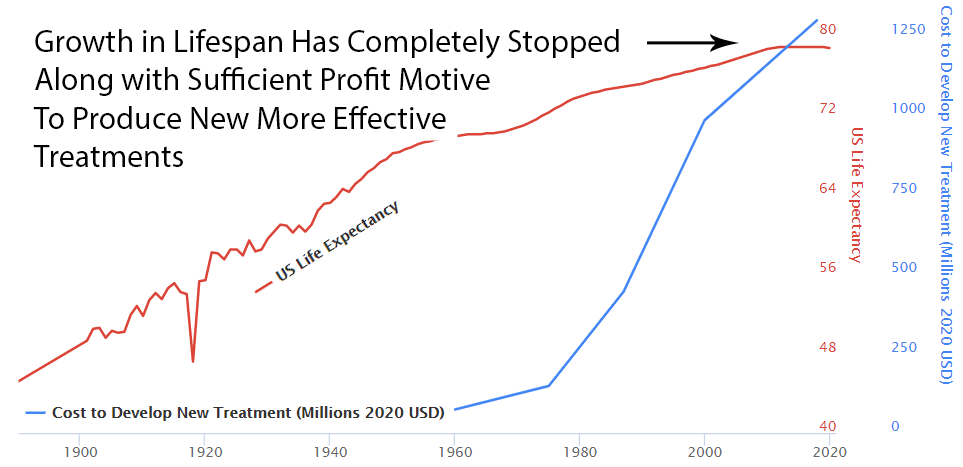
The new regulatory clampdown on approvals immediately reduced the production of new treatments by 70%.
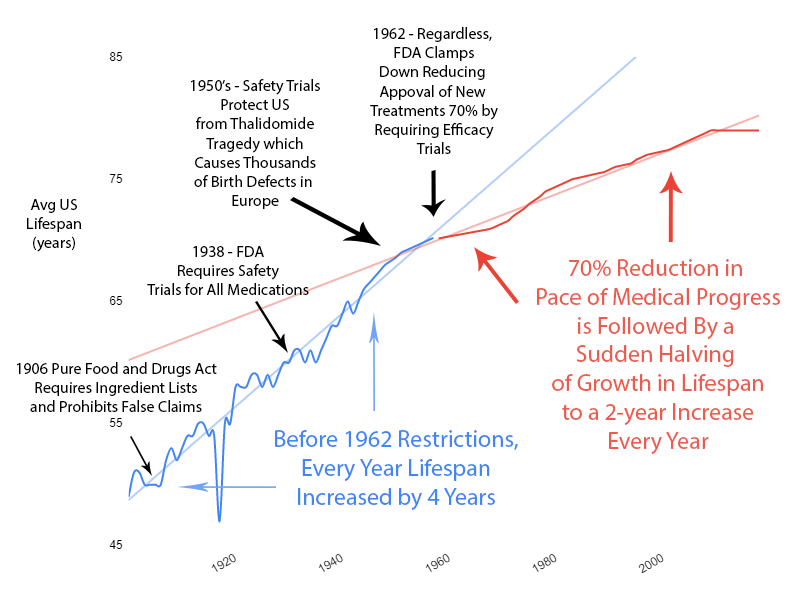
Slowed Growth in Lifespan
Over the previous 50 years, rapid advancements in medical science had been producing a 4-year increase in human lifespan every year. This amazingly linear growth rate had followed millennia with a flat human lifespan of around 28 years. Following this new 70% reduction in the pace of medical progress, the growth in human lifespan was immediately cut in half to an increase of 2 years per year.
Diminishing Returns?
You might say “It seems more likely — or as likely — to me that drug development provides diminishing returns to life expectancy.” However, diminishing returns produce a slope of exponential decay. It may be partially responsible, but it’s not going to produce a sudden change in the linear slope of a curve a linear as life expectancy was before and after the FDA.
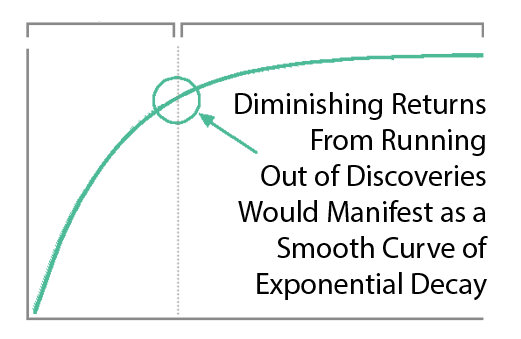
Additionally, we’re only 3 lifetimes from George Washington. The modern scientific method has only been systematically applied to medicine for like .0001% of human history. However, the more clinical research studies I read, the more I realize we don’t know. We know basically nothing at this point compared to what will eventually be known about the human body. And the currently highly restrictive overly cautious method of clinical research prevents us from knowing more faster. We’re at the very beginning of thousands or millions of years of systematic discovery. So it’s unlikely that this decline in lifespan growth is the result of diminishing returns due to our running out of things to discover.
2 Year Drug Lag
It currently takes over 10 years for a life-saving drug to make its way through the FDA’s regulatory process and become available to dying patients.
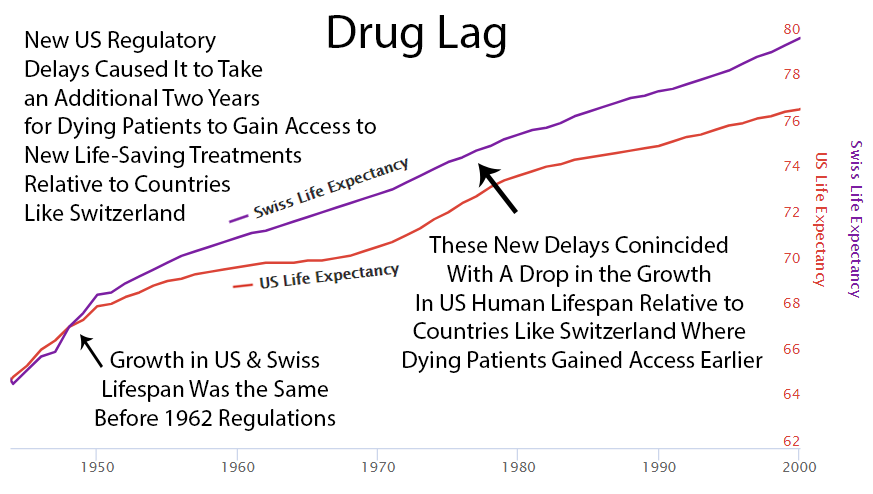
Perhaps it’s a coincidence, but you can see an increase in drug approvals in the 80’s and at the same time the gap between Switzerland and the US gets smaller then. Then US approvals go back down in the 90’s and the gap expands again.
Here’s a news story from the Non-Existent Times by No One Ever without a picture of all the people that die from lack of access to life-saving treatments that might have been.
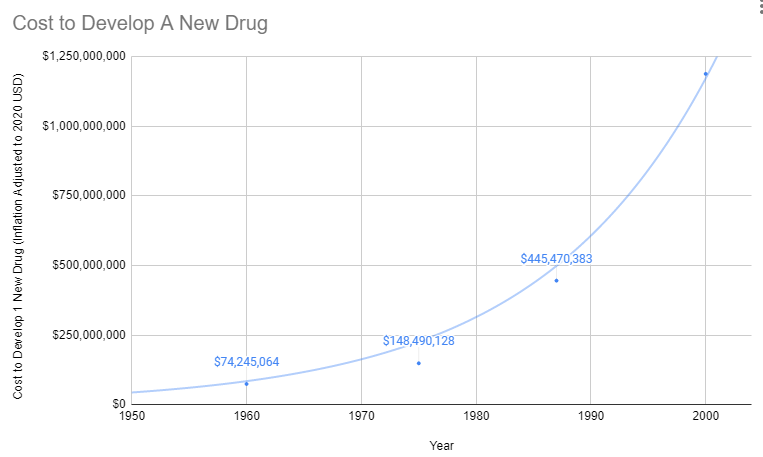
Human Costs of the High Cost of Clinical Research
Since 1962, the cost of bringing a new treatment to market has gone from $74 million to over $1 billion US dollars (2020 inflation-adjusted).
Treatments for Rare Diseases Make No Financial Sense
The costs of FDA regulations do not vary with the number of potential users of the drug, so the decline in drug development has been especially important in the treatment of rare diseases. By definition, each rare disease afflicts only a small number of people, but there are thousands of rare diseases. In aggregate, rare diseases afflict millions of Americans: according to an AMA estimate (AMA 1995), as many as 10% of the population. Thus, millions of Americans have few or no therapies available to treat their diseases because of increased costs of drug development brought about by stringent FDA “safety and efficacy” requirements. In response to this problem, in 1983 the Orphan Drug Act was passed to provide tax relief and exclusive privileges to firms developing drugs for diseases affecting two hundred thousand or fewer Americans (AMA 1995). It would be better to reduce or eliminate FDA regulations for all drugs and patient populations.
Oligopolies Protect Existing Inferior Treatments
Prior to 1962, a brilliant scientist could come up with a new treatment, raise $74 million dollars for safety and efficacy testing, and bring it to market. With the current cost of bringing a new treatment to market over a billion dollars, there are only a handful of companies with enough money to risk a billion with a 90% chance of rejection by the FDA. So today the brilliant scientist goes to one of these companies and the company buys the patent for several million dollars. It’s likely the drug company already has an existing inferior drug on the market that they’ve already spent a billion dollars getting approved.
Then the drug company has 2 options:
Option 1: Risk $1 billion on clinical trials
Possibility A: Drug turns out to be one of the 90% the FDA rejects. GIVE BANKER A BILLION DOLLARS. DO NOT PASS GO.
Possibility B: Drug turns out to be one of the 10%, the FDA approves. Yay!!! Now it’s time to try to recover that billion dollars. However, there are very few drug companies with enough money to survive this game. So, this company almost certainly already has an existing crappier drug on the market to treat the same condition. Hence, any profit they make from this drug will likely be subtracted from revenue from existing inferior drugs they’ve already spent a billion dollars on.
Option 2: Put the patent on the shelf
Do not take a 90% chance of wasting a billion dollars on failed trials. Do not risk making your already approved cash-cow drugs obsolete.
What’s the benefit of bringing better treatment to market if you’re just going to lose a billion dollars? Either way, the profit incentive is entirely in favour of just buying better treatments and shelving them.
With the incentives completely stacked against the discovery of new treatments, it’s amazing there’s any medical progress whatsoever. In fact, a complete stoppage in medical progress is exactly what we’ve seen if it’s measured by lifespan.
Efficacy Trials Before 1962
There are hundreds of conditions that a given drug may be effective at treating. However, it’s impossible to know which conditions a drug may improve until it is tried by real-world patients.
Prior to the 1962 regulations, it cost a drug manufacturer an average of $74 million dollars (2020 inflation-adjusted) to develop and test a new drug for safety before bringing it to market. Once the FDA had approved it as safe, efficacy testing was performed by the third-party American Medical Association.
When all other treatments had failed, the 144,000 Physicians in the American Medical Association were allowed to offer suffering or dying patients the option of trying proven-safe treatments for which the effectiveness was not yet known.
The AMA would collect data from its 144,000 physicians members on the efficacy of different drugs for various conditions. Once it was determined which conditions a drug was or was not effective for, the results would be published in the Journal of the American Medical Association (JAMA). The AMA would only give its seal to drugs which it had determined were safe and effective for specific conditions.
The 1962 regulations made these large real-world efficacy trials illegal. Ironically, despite the fact that the new regulations were primarily focused on ensuring that drugs were effective through controlled FDA efficacy trials, they massively reduced the quantity and quality of the efficacy data that was collected for several reasons:
- New Trials Were Much Smaller
- Were Far More Expensive
- Participants Were Less Representative of Actual Patients
- They Were Run by Drug Companies with Conflicts of Interest Instead of the 3rd Party AMA
Exclusion Criteria
Subjects in FDA Trials Are Not Representative of the Actual People Receiving Treatment
External validity is the extent to which the results can be generalized to a population of interest. The population of interest is usually defined as the people the intervention is intended to help.
Phase III clinical trials are designed to exclude a vast majority of the population of interest. In other words, the subjects of the drug trials are not representative of the prescribed recipients, once said drugs are approved. One investigation found that only 14.5% of patients with major depressive disorder fulfilled eligibility requirements for enrollment in an antidepressant efficacy trial.
As a result, the results of these trials are not necessarily generalizable to patients matching any of these criteria:
- Suffer from multiple mental health conditions (e.g. post-traumatic stress disorder, generalized anxiety disorder, bipolar disorder, etc.)
- Engage in drug or alcohol abuse
- Suffer from mild depression (Hamilton Rating Scale for Depression (HAM-D) score below the specified minimum)
- Use other psychotropic medications
These facts call into question the external validity of standard efficacy trials.
Impossible to Discover Effectiveness for All Conditions
The fact that co-morbidities are excluded also makes it impossible to discover potential new uses for a treatment.
Sources
New Trials Were Very Small
Millions of people are prescribed medications based on efficacy trials with as few as 20 participants.
Due to exclusion criteria and added cost, patient sample sizes are very small. The number of subjects per trial on average is:
- 275 patients are sought per cardiovascular trial
- 20 patients per cancer trial
- 70 patients per depression trial
- 100 per diabetes trial
You can look up the efficacy and trial size for any drug at https://dailymed.nlm.nih.gov. On a drug page, scroll down to the “CLINICAL STUDIES” section to see the efficacy tables. Here’s the data for Immediate-Release Bupropion (Wellbutrin).
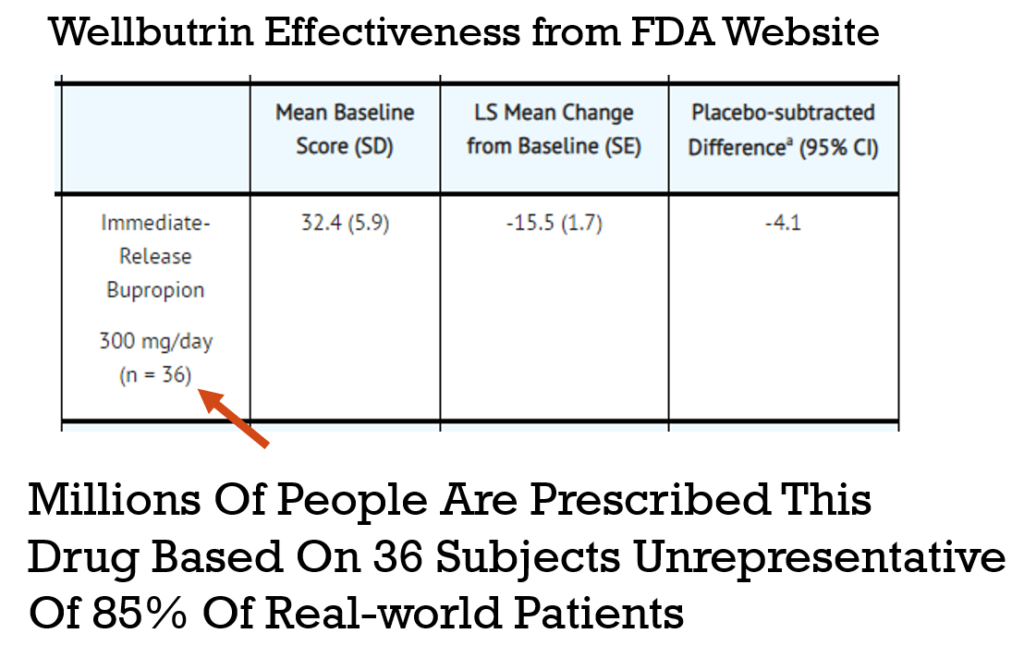
Solution: Collect Quantified Self Data on Actual Patients
In the real world, no patient can be excluded. Even people with a history of drug or alcohol abuse, people on multiple medications, and people with multiple conditions must be treated. Only through the crowdsourcing of this research, would physicians have access to the true effectiveness rates and risks for their real world patients.
The results of crowdsourced studies would exhibit complete and utter external validity since the test subjects are identical to the population of interest.
Sources
- Google Spreadsheet of FDA Spending vs Life-Expectancy
- Summary of NDA Approvals & Receipts, 1938 to the present
- Theory, Evidence, and Examples of FDA Harm
- DATA
- GDP
- Reform, Regulation, and Pharmaceuticals — The Kefauver–Harris Amendments at 50
- Consumer Price Index
- Estimates of World GDP, One Million B.C. – Present
- Newspaper Generator
- Report suggests drug-approval rate now just 1-in-10
- How many people die and how many are born each year?
- Gross World Product per capita
- History of Clinical Trials
- How many life-years have new drugs saved?
- CATO
- Medical Innovation
- Timeline History of Clinical Research
- FDA and Clinical Drug Trials: A Short History
- Do Off-Label Drug Practices Argue Against FDA Efficacy Requirements?
- Reform Options
- Before Occupy: How AIDS Activists Seized Control of the FDA in 1988
- A Brief History of the Center for Drug Evaluation and Research
- Milestones in U.S. Food and Drug Law History

Leave a Reply