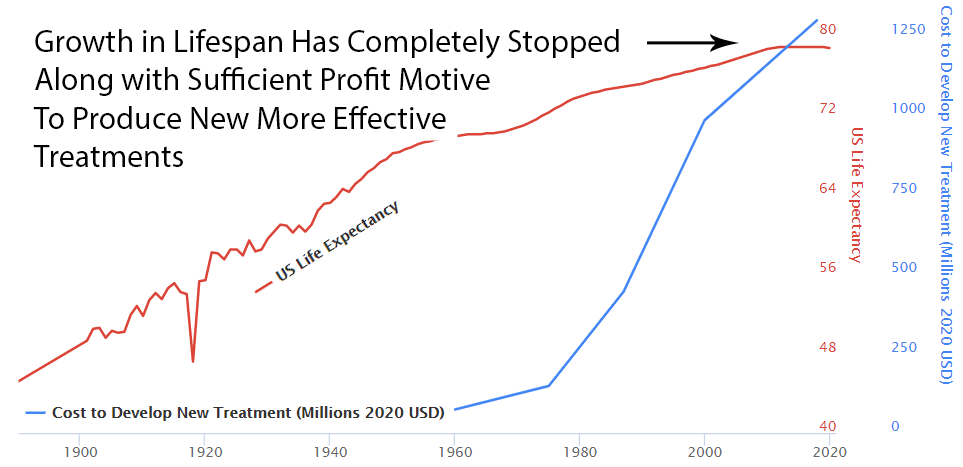
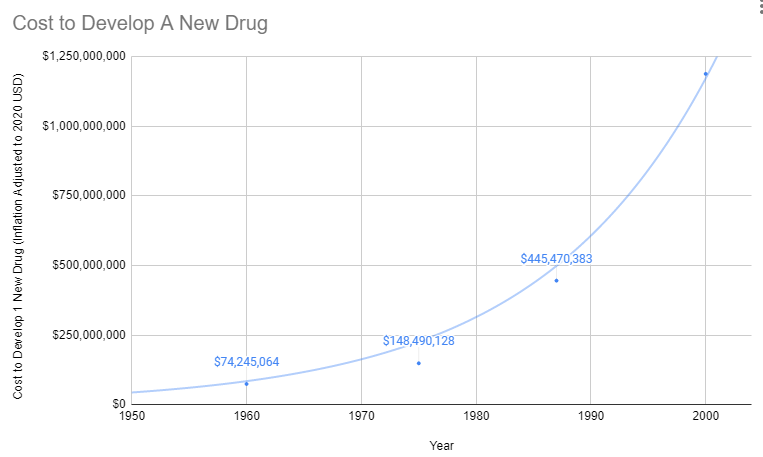
Since 1962, the cost of bringing a new treatment to market has gone from $74 million to over $1 billion US dollars (2020 inflation-adjusted).
Treatments for Rare Diseases Make No Financial Sense
The costs of FDA regulations do not vary with the number of potential users of the drug, so the decline in drug development has been especially important in the treatment of rare diseases. By definition, each rare disease afflicts only a small number of people, but there are thousands of rare diseases. In aggregate, rare diseases afflict millions of Americans: according to an AMA estimate (AMA 1995), as many as 10% of the population. Thus, millions of Americans have few or no therapies available to treat their diseases because of increased costs of drug development brought about by stringent FDA “safety and efficacy” requirements. In response to this problem, in 1983 the Orphan Drug Act was passed to provide tax relief and exclusive privileges to firms developing drugs for diseases affecting two hundred thousand or fewer Americans (AMA 1995). It would be better to reduce or eliminate FDA regulations for all drugs and patient populations.
Oligopolies Protect Existing Inferior Treatments
Prior to 1962, a brilliant scientist could come up with a new treatment, raise $74 million dollars for safety and efficacy testing, and bring it to market. With the current cost of bringing a new treatment to market over a billion dollars, there are only a handful of companies with enough money to risk a billion with a 90% chance of rejection by the FDA. So today the brilliant scientist goes to one of these companies and the company buys the patent for several million dollars. It’s likely the drug company already has an existing inferior drug on the market that they’ve already spent a billion dollars getting approved.
Then the drug company has 2 options:
Option 1: Risk $1 billion on clinical trials
Possibility A: Drug turns out to be one of the 90% the FDA rejects. GIVE BANKER A BILLION DOLLARS. DO NOT PASS GO.
Possibility B: Drug turns out to be one of the 10%, the FDA approves. Yay!!! Now it’s time to try to recover that billion dollars. However, there are very few drug companies with enough money to survive this game. So, this company almost certainly already has an existing crappier drug on the market to treat the same condition. Hence, any profit they make from this drug will likely be subtracted from revenue from existing inferior drugs they’ve already spent a billion dollars on.
Option 2: Put the patent on the shelf
Do not take a 90% chance of wasting a billion dollars on failed trials. Do not risk making your already approved cash-cow drugs obsolete.
What’s the benefit of bringing better treatment to market if you’re just going to lose a billion dollars? Either way, the profit incentive is entirely in favour of just buying better treatments and shelving them.
With the incentives completely stacked against the discovery of new treatments, it’s amazing there’s any medical progress whatsoever. In fact, a complete stoppage in medical progress is exactly what we’ve seen if it’s measured by lifespan.